
All elements ( up to Z=60 ) are determined simultaneously by increasing the analytical speed. Direct spectrophotometer (simultaneous multielement spectrophotometer)ĭirect spectrometers are faster, more precise, and more accurate than sequential ones.

ICP source comprises three concentric silica quartz tubes each of which is open at the top. This temperature is enough for the ionization of samples. ICP source is extremely hot and produces a maximum temperature of up to 6500 °K. Electrically heated graphite tubes are used instead of flame for atomizing purposes in electrothermal atomizers. It is also known as a graphite furnace atomizer. The different types of burners used for atomizing purposes areĮlectrothermal atomizers are also used for the atomization of samples.
#Atomic emission spectrum scarf free#
Atomizers are used for the conversion of solid or liquid samples to free gaseous atoms.įlame atomizers require oxidant or flame gases. AtomizerĪtomizers consist of flames and burners. Argon gas flowing at very high pressure is used for changing liquid to an aerosol spray. The sample is forced into a mixing chamber at a flow rate of 1 mL /min by a peristaltic pump and nebulized by the steam of argon flowing at about 1L /min. The most commonly used nebulizers are concentric glass nebulizers and cross-flow nebulizers. Various types of nebulizers are used for carrying samples to the plasma flame section. The sample is introduced into plasma with the help of nebulizers. Atomic emission spectroscopy is a useful technique for determining the inorganic constituents in different samples and much more.Ī cup-shaped graphite electrode acts as a sample cell in atomic emission spectroscopy. This was one of the best techniques used at that time for quantitative analysis. This technique was developed by Sir Norman Lockyer from the United Kingdom, although it was Henrick Lundegardn who pioneered it. It is called OES due to the optical property of radiation during the de-excitation process.ĭevelopment of inductively coupled plasma atomic emission spectroscopy (ICP-AES) When an electron emits electromagnetic radiation while coming back from an excited to de-excited state, the EMR is measured and analyzed. It involves the excitation and de-excitation processes for electrons by absorption of radiation. It is named altogether as inductively coupled plasma atomic emission spectroscopy (ICP-AES) or inductive coupled plasma optical emission spectrometry (ICP-OES). Atomic emission spectroscopy involves both excitation (absorption of radiation) and de-excitation (emission of radiation) of electrons.Ītomic emission spectroscopy, also known as ICP spectroscopy stands for inductively coupled plasma.

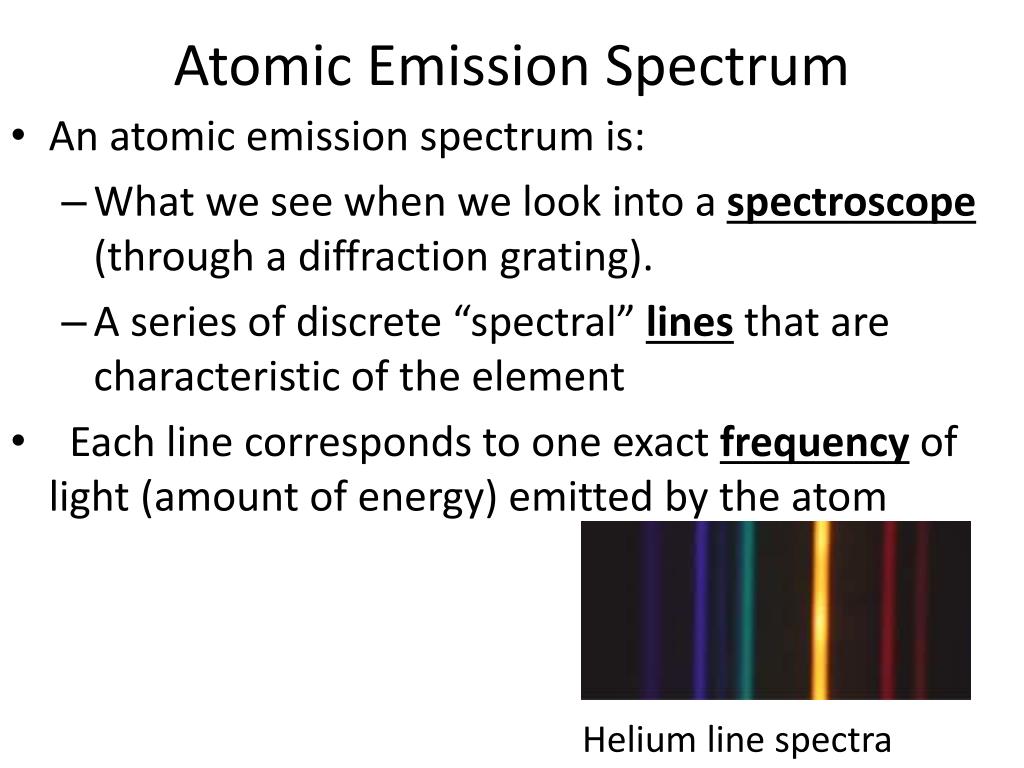
When an excited atom returns to the ground level, it emits radiation in a discrete wavelength. Atomic emission spectroscopy (AES) is an analytical technique used for the quantification of metal atoms by measuring the intensity of light emitted by the atoms in excited states.


 0 kommentar(er)
0 kommentar(er)
